About New Generation Sequencing
Massive parallel sequencing or massively parallel sequencing is any of several high-throughput approaches to DNA sequencing using the concept of massively parallelprocessing; it is also called next-generation sequencing (NGS) or second-generation sequencing. Some of these technologies emerged in 1994-1998 and have been commercially available since 2005. These technologies use miniaturized and parallelized platforms for sequencing of 1 million to 43 billion short reads (50-400 bases each) per instrument run.
Many NGS platforms differ in engineering configurations and sequencing chemistry. They share the technical paradigm of massive parallel sequencing via spatially separated, clonally amplified DNA templates or single DNA molecules in a flow cell. This design is very different from that of Sanger sequencing—also known as capillary sequencing or first-generation sequencing—that is based on electrophoretic separation of chain-termination products produced in individual sequencing reactions.
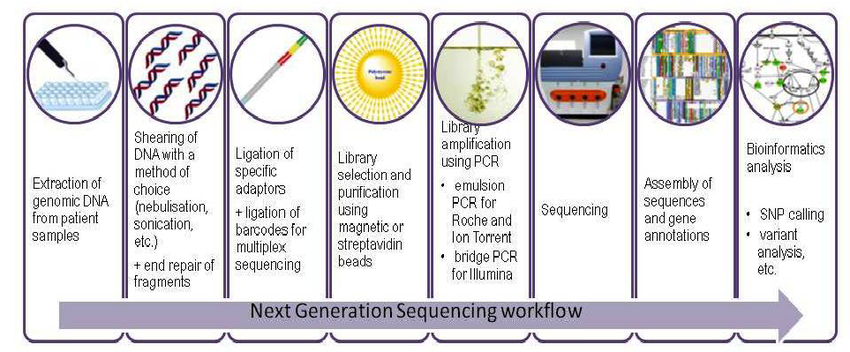
DNA sequencing with commercially available NGS platforms is generally conducted with the following steps. First, DNA sequencing libraries are generated by clonal amplification by PCR in vitro. Second, the DNA is sequenced by synthesis, such that the DNA sequence is determined by the addition of nucleotides to the complementary strand rather than through chain-termination chemistry.
Bioinformatics analyses are used to piece together these fragments by mapping the individual reads to the human reference genome. Each of the three billion bases in the human genome is sequenced multiple times, providing high depth to deliver accurate data and an insight into unexpected DNA variation. NGS can be used to sequence entire genomes or constrained to specific areas of interest, including all 22 000 coding genes (a whole exome) or small numbers of individual genes.
Ion semiconductor sequencing is a method of DNA sequencing based on the detection of hydrogen ions that are released during the polymerization of DNA. This is a method of "sequencing by synthesis", during which a complementary strand is built based on the sequence of a template strand.
A microwell containing a template DNA strand to be sequenced is flooded with a single species of deoxyribonucleotide triphosphate (dNTP). If the introduced dNTP is complementary to the leading template nucleotide, it is incorporated into the growing complementary strand. This causes the release of a hydrogen ion that triggers an ISFET ion sensor, which indicates that a reaction has occurred. If homopolymer repeats are present in the template sequence, multiple dNTP molecules will be incorporated in a single cycle. This leads to a corresponding number of released hydrogens and a proportionally higher electronic signal.
This technology differs from other sequencing technologies in that no modified nucleotides or optics are used. Ion semiconductor sequencing may also be referred to as Ion Torrent sequencing, pH-mediated sequencing, silicon sequencing, or semiconductor sequencing.
Technology development history
The technology was licensed from DNA Electronics Ltd, developed by Ion Torrent Systems Inc. and was released in February 2010. Ion Torrent have marketed their machine as a rapid, compact and economical sequencer that can be utilized in a large number of laboratories as a bench top machine.
Potential uses of NGS in clinical practice
NGS captures a broader spectrum of mutations than Sanger sequencing. The spectrum of DNA variation in a human genome comprises small base changes (substitutions), insertions and deletions of DNA, large genomic deletions of exons or whole genes and rearrangements such as inversions and translocations. Traditional Sanger sequencing is restricted to the discovery of substitutions and small insertions and deletions. For the remaining mutations dedicated assays are frequently performed, such as fluorescence in situ hybridisation (FISH) for conventional karyotyping, or comparative genomic hybridisation (CGH) microarrays to detect submicroscopic chromosomal copy number changes such as microdeletions. However, these data can also be derived from NGS sequencing data directly, obviating the need for dedicated assays while harvesting the full spectrum of genomic variation in a single experiment. The only limitations reside in regions which sequence poorly or map erroneously due to extreme guanine/cytosine (GC) content or repeat architecture, for example, the repeat expansions underlying Fragile X syndrome, or Huntington's disease.
Genomes can be interrogated without bias
Capillary sequencing depends on preknowledge of the gene or locus under investigation. However, NGS is completely unselective and used to interrogate full genomes or exomes to discover entirely novel mutations and disease causing genes. In paediatrics, this could be exploited to unravel the genetic basis of unexplained syndromes. Allying these molecular data with detailed clinical phenotypic information has been successful in identifying novel genes mutated in affected children with similar clinical features.
The increased sensitivity of NGS allows detection of mosaic mutationsMosaic mutations are acquired as a postfertilisation event and consequently they present at variable frequency within the cells and tissues of an individual. Capillary sequencing may miss these variants as they frequently present with a subtlety which falls below the sensitivity of the technology.
NGS sequencing provides a far more sensitive read-out and can therefore be used to identify variants which reside in just a few per cent of the cells, including mosaic variation. In addition, the sensitivity of NGS sequencing can be increased further, simply by increasing sequencing depth. This has seen NGS employed for very sensitive investigations such as interrogating foetal DNA from maternal blood or tracking the levels of tumour cells from the circulation of cancer patients.
Microbiology
The main utility of NGS in microbiology is to replace conventional characterisation of pathogens by morphology, staining properties and metabolic criteria with a genomic definition of pathogens. The genomes of pathogens define what they are, may harbour information about drug sensitivity and inform the relationship of different pathogens with each other which can be used to trace sources of infection outbreaks.
Oncology
The fundamental premise of cancer genomics is that cancer is caused by somatically acquired mutations, and consequently it is a disease of the genome. With the advent of NGS, cancer genomes can now be systemically studied in their entirety, an endeavour ongoing via several large scale cancer genome projects around the world, including a dedicated paediatric cancer genome project. Individual cancer sequencing may, therefore, provide the basis of personalised cancer management.
Oncomine Platform Software provides solutions for individual researchers as well as collaborative consortia, with robust, peer-reviewed analysis methods and a powerful set of analysis functions that compute gene expression signatures, clusters, and gene-set modules, automatically extracting biological insights from the data.
A widely-used tool, the software is cited in more than 880 peer-reviewed journal articles and has been used as a foundation for ground-breaking discoveries; its unique features include:
- Scalability—with >700 independent datasets
- High quality—with expertly curated data
- Consistency—with a rich, extensive, and controlled ontology of terms
- Standardized analysis—with conventions that assure clear and consistent interpretation of results
